A Journey to a Cure
 Developing a cure or treatment is a long, complicated and expensive process. No one knows how long it will take, but we will not give up until we have found a cure. We are not there yet, but we are getting so much closer. Please have hope. Although a cure may not be found in your child’s lifetime, research done now will help future children affected by these devastating diseases. We need to continue fundraising to help researchers continue to do the vital studies required before more clinical trials can commence. We need patients to enroll in natural history studies, and to consider enrolling in clinical trials and other studies, if they are appropriate.
Developing a cure or treatment is a long, complicated and expensive process. No one knows how long it will take, but we will not give up until we have found a cure. We are not there yet, but we are getting so much closer. Please have hope. Although a cure may not be found in your child’s lifetime, research done now will help future children affected by these devastating diseases. We need to continue fundraising to help researchers continue to do the vital studies required before more clinical trials can commence. We need patients to enroll in natural history studies, and to consider enrolling in clinical trials and other studies, if they are appropriate.
This page will give you a basic understanding of the research process. The NTSAD Scientific Advisory Committee closely monitors the scientific field and alerts NTSAD Families of important breakthroughs and developments. To learn more about which stage the research is at for each disease please visit Research by Disease.
Natural History Studies
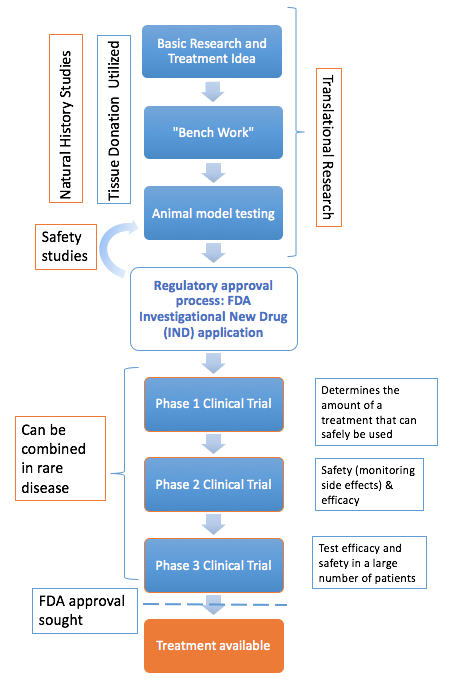
Natural history studies study the course of a disease. They are essential in rare disease research. There are no risks to the patients, and the research will benefit the entire disease community. Data collected from natural history studies is garnering importance as success in research moves us closer to initiating clinical trials. It is vital to have a baseline knowledge of the disease progression against which to measure the success of therapy. Researchers are conducting these vital natural history studies and collecting fundamental data to inform clinical trials. Furthermore, natural history studies provide valuable information to families and clinicians to help manage these difficult diseases. Natural history work is ongoing for many of our diseases with the aim of funding more natural history studies in preparation for future clinical trials.
Biomarker Studies
Biomarker Studies are ways to objectively measure disease severity to better understand how a potential therapy may be helping. A biomarker may be a measurable substance such as the substrate accumulated in the disease or it may be imaging, such as an MRI, that shows disease improvement
Basic Research
Before scientists can attempt to treat disease they must understand the exact cause of the disease. How are cells damaged by the missing enzyme? What are the biochemical pathways leading to disease? Basic research is often called the "How does that work?" stage. Researchers will then create a theory about how a treatment could work, and test these theories in the lab.
Pre-Clinical Development
Translational Research
The next step is to take the basic understanding of the disease and try to intervene to slow, stop or reverse the symptoms. Often these studies start in a culture dish but eventually almost always result in animal model studies. The first step in animal studies is finding an animal that can serve as a model for the disease. This often involves genetically modifying the animal so that it has the disease. Sandhoff mice are the most commonly used Tay-Sachs and Sandhoff animal model. Both mouse and rat models are commonly used to study Canavan disease. Affected mice are given potential treatments and closely monitored for improvements. If the mice show positive results, studies in larger animals such as cats and sheep are done because their brains are closer in size to humans.
Regulatory Approval Process
After a potential therapy has been thoroughly demonstrated to alleviate disease in the animal models the regulatory phase of preclinical developments begins. Clinical trials must be reviewed and allowed to proceed by the Food and Drug Administration (FDA). This can be a lengthy and complicated process in itself. The steps in FDA clinical trial approval include:
- Pre-IND meetings with FDA
- Toxicology studies in animals
- Producing the treatment (e.g. vector) using Good Manufacturing Practices
- Investigational Drug Application (IND)
In addition to FDA review of the clinical trial IND, the researchers must also receive approval from the Institutional Review Board (IRB) of all clinical trials sites. Also review and public discussion of the clinical trial protocol by the NIH Recombinant DNA Advisory Committee (RAC) may be necessary.
Clinical Trials
Clinical trials are typically done in three phases. However, when looking at treatments for very rare diseases like Tay-Sachs and other related diseases, the phases are often modified and blended together. Phase I typically looks at safety, Phase II looks at efficacy and safety, and Phase III verifies the treatment is safe and effective in a larger number of clinical trial subjects. Comprehensive natural history studies and clear biomarkers are vital to clinical trials to measure the efficacy of the treatment. The FDA approval of the product is necessary before the therapy is commercially available.
Off Label Use Studies
After clinical trials, the product receives FDA approval to be used as a treatment for the specific disease population that it was tested in. However, physicians may choose to use the treatment in a different population. This is called “off label use.”

