The Two General Categories
 NTSAD is dedicated to leading the worldwide fight to treat and cure Tay-Sachs, Canavan, GM1, and Sandhoff diseases. Treating these diseases falls into two general categories: (1) Restoring the missing enzyme or (2) Decreasing the waste accumulation. These diseases are especially difficult to treat because they affect the central nervous system (CNS) which is protected by the blood brain barrier. The scientific community generally agrees that no single therapy will cure these diseases but that rather a combination of approaches will slow, halt and even reverse the damage caused by these diseases. This section provides a basic introduction into these potential therapies.
NTSAD is dedicated to leading the worldwide fight to treat and cure Tay-Sachs, Canavan, GM1, and Sandhoff diseases. Treating these diseases falls into two general categories: (1) Restoring the missing enzyme or (2) Decreasing the waste accumulation. These diseases are especially difficult to treat because they affect the central nervous system (CNS) which is protected by the blood brain barrier. The scientific community generally agrees that no single therapy will cure these diseases but that rather a combination of approaches will slow, halt and even reverse the damage caused by these diseases. This section provides a basic introduction into these potential therapies.
Frequently Asked Questions
Category 1: Restoring Enzyme Function
The majority of research and potential therapies focus on restoring the enzymes because the missing or deficient enzymes are the primary pathology, or disease causing mechanism, in most of these diseases.
Gene Therapy
Gene therapy has a lot of promise to cure genetic diseases
The goal of gene therapy is introduce the correct genetic code into a cell so that enzyme production can occur. The correct genetic code may be delivered in various ways, but the most common approach is using a viral vector. Typically, when a virus enters the human body, it injects its own genetic material into the nucleus of the human cell, so the viral genetic material will be then be replicated in the human body when the cell divides. In gene therapy, the DNA of the non-disease causing virus is altered so that it carries the correct genetic code which the person needs. Therefore, when the virus enters the cell and injects the genetic material into the cell's nucleus, the cell will be given the correct information to produce the enzyme which it is missing.
There are many challenges to safe and effective gene therapy. Some of these include
- difficulty of creating effective vectors for gene delivery to the brain
- need to introduce the gene into a large number of cells to be effective
- challenge of inserting the genes into the appropriate brain cells
- potential for an oncogenic (cancer) event to occur as a result of the insertion of the gene into the cell's chromosomes
However, this is an exciting and progressing research area.
Adeno-associated viruses (AAV) are an example of a viral vector which is showing promising results in gene therapy trials in animal models. It is a harmless virus which most people carry, so is believed to be a safe way to insert new genetic material into humans. Read more about the delivery approaches here.
Research into gene therapy in animals with Tay Sachs and Canavan has also been using the AAV gene therapy, as is described on the Research by Disease page.
Learn more:
http://ghr.nlm.nih.gov/handbook/therapy/genetherapy
http://learn.genetics.utah.edu/content/genetherapy/
Pharmacological/Molecular Chaperone Therapy
Pharmacological or Molecular Chaperones are small molecules that are able to cross the blood brain barrier into the central nervous system. These chaperones attach to an inactive enzyme so that it takes the correct functional shape. However, chaperones only work with certain mutations, and have the potential to reduce enzyme function if given at the wrong dose
Enzyme Replacement Therapy
Enzyme Replacement Therapy (ERT) provides the missing or deficient enzyme through regular IV infusions. However, enzymes are large molecules that do not pass the blood brain barrier into the central nervous system. Approved ERT therapies are currently only effective for treating the non-neurological symptoms of diseases.
Stem Cell Therapy
Stem cells differ from other kinds of cells in the body. All stem cells - regardless of their source - have three unique properties:
- Capable of dividing and renewing themselves for long periods of time
- Unspecialized
- Can give rise to specialized cells
These unique properties could be used for cell-based therapies in which stem cells that are genetically altered to produce the missing enzyme are delivered to the brain or central nervous system.
Stem cell therapy has a lot of promise to cure but faces formidable challenges to develop safe and effective therapies. Some of the challenges include:
- Risk of an immune response leading to rejection of these cells
- Risk of cells differentiating in an unexpected way
- Transmission of donor-related diseases that reside in those stem cells
- Ability to scale-up the amount of cells needed for humans
- Acceptance of this approach owing to the controversy over embryonic stem cells
The embryonic stem cell controversy is becoming less of an issue as scientists have recently discovered how to manipulate adult cells into a stem cell state but more work is necessary to understand their therapeutic properties and potential.
Bone Marrow Transplant
A bone marrow transplant (BMT), replaces an affected person's bone marrow with donor marrow that can produce the missing enzyme. The procedure is risky, invasive and has had very little success in Tay-Sachs, Sandhoff and GM-1. If done early enough it can be effective in slowing and even stopping the disease in other allied diseases like Krabbe and MPS.Bone Marrow Transplant is very invasive. Children often succumb to complications of the procedure. The immune system must be completely destroyed before introducing the healthy bone marrow. It is unclear how many of the healthy stem cells cross into the central nervous system.
Stop Codon Readthrough
Stop-Codon Readthrough Technology is a field that could potentially treat genetic diseases caused by stop codons. Stop codons are genetic phrases that indicate the end of the gene, like the period at the end of a sentence. Some genetic diseases are caused by mutations which place the period before the end of the gene resulting in proteins (including enzymes) that do not work properly. Stop Codon Read Through Technology uses small molecules that cross the blood brain barrier into the central nervous system and induce the cell to ignore the stop codon resulting in a properly functioning enzyme. One challenge of this technology is learning how to effectively use this technology to both read through disease causing stop codons and ignore stop codons in the proper place.
Category 2: Substrate Reduction Therapy
Reducing waste accumulation via substrate reduction therapy is another strategy to treat Tay-Sachs, Sandhoff, GM-1, Canavan and related diseases. It does not have the same potential to cure the disease because it does not address the primary disease causing mechanism, the missing or deficient enzyme. However, if successful it could slow or stop the disease progression giving the most precious gift - time.
How does substrate reduction therapy work?
Substrate reduction therapy uses small molecules that pass the blood brain barrier into the central nervous system and decrease the amount of substrate or waste product that accumulates. Think of a clogged sink with the faucet running and turning down the faucet (c). The water will still fill up the sink but it will take longer.
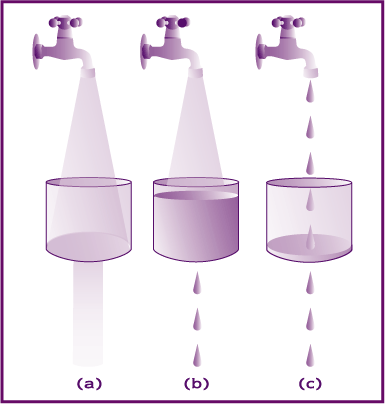
A visual representation:
a) In most individuals the substrate (water) can be degraded efficiently by adequate enzyme volume (hole in sink).
b) In affected individuals the amount of enzyme is insufficient to efficiently degrade the substrate and it accumulates.
c) In affected individuals treated with substrate synthesis reduction therapy, the amount of substrate is decreased to match the amount of residual enzyme to prevent accumulation.
The NTSAD Scientific Advisory Committee (SAC) subcommittee on experimental therapies recently reviewed the data regarding miglustat’s safety and potential efficacy. Read this Report on Substrate Reduction Therapy for Lysosomal Storage Diseases.
How Research is Used
To find out how these techniques are currently being used in research, visit the Research by Disease.


